Key Highlights:
- Verastem Oncology's new drug targets the KRAS G12D mutation with FDA approval.
- Analysts predict a significant upside potential for Verastem, estimating an average target price of $13.00.
- The company's brokerage recommendation reflects an "Outperform" status, suggesting positive market sentiment.
Verastem Oncology's Breakthrough: FDA Approval
Verastem Oncology (VSTM, Financial) has achieved a significant milestone by securing FDA approval for its investigational drug, VS-7375. This oral therapy is designed to target the KRAS G12D mutation, a key player in tumor growth. The company is preparing to initiate a Phase 1/2a clinical trial by mid-2025, focusing on advanced solid tumors associated with this mutation.
Analyst Price Targets and Market Predictions
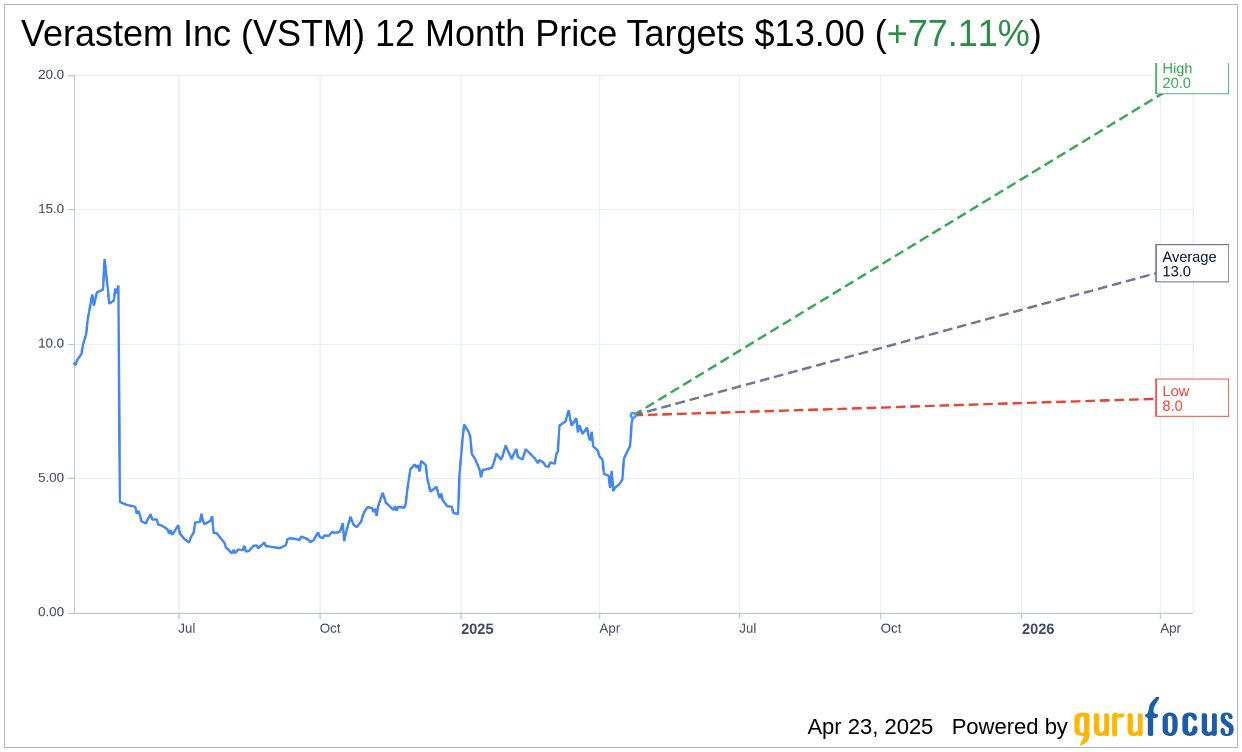
According to estimates from eight analysts, Verastem Inc (VSTM, Financial) has a promising future with an average price target of $13.00. Projections range from a high of $20.00 to a low of $8.00, reflecting an average potential upside of 82.71% from the current share price of $7.12. Investors can find more detailed analyses on the Verastem Inc (VSTM) Forecast page.
Brokerage Recommendations: A Positive Outlook
Market confidence in Verastem Inc (VSTM, Financial) remains robust, as indicated by the consensus recommendation from nine brokerage firms. With an average brokerage recommendation of 1.7, the stock is rated as "Outperform." This rating uses a scale from 1 to 5, where 1 represents a "Strong Buy" and 5 indicates a "Sell," signaling overall positive expectations from market analysts.
